when a high-energy bond of atp is broken, primarily what happens to the released energy?
6.4: ATP: Adenosine Triphosphate
- Page ID
- 1853
Skills to Develop
- Explicate the role of ATP as the cellular energy currency
- Describe how energy is released through hydrolysis of ATP
Even exergonic, energy-releasing reactions require a small corporeality of activation free energy in lodge to proceed. However, consider endergonic reactions, which require much more than energy input, considering their products have more than free energy than their reactants. Within the cell, where does energy to power such reactions come from? The answer lies with an energy-supplying molecule chosen adenosine triphosphate, or ATP. ATP is a small, relatively unproblematic molecule (Figure \(\PageIndex{1}\)), but within some of its bonds, information technology contains the potential for a quick burst of energy that can be harnessed to perform cellular work. This molecule tin be thought of as the main energy currency of cells in much the same mode that coin is the currency that people substitution for things they demand. ATP is used to power the bulk of free energy-requiring cellular reactions.

As its name suggests, adenosine triphosphate is comprised of adenosine bound to three phosphate groups (Figure \(\PageIndex{ane}\)). Adenosine is a nucleoside consisting of the nitrogenous base adenine and a five-carbon sugar, ribose. The 3 phosphate groups, in lodge of closest to furthest from the ribose sugar, are labeled alpha, beta, and gamma. Together, these chemic groups constitute an energy powerhouse. Still, not all bonds within this molecule exist in a especially high-energy state. Both bonds that link the phosphates are equally loftier-free energy bonds (phosphoanhydride bonds) that, when broken, release sufficient energy to power a variety of cellular reactions and processes. These high-energy bonds are the bonds betwixt the second and tertiary (or beta and gamma) phosphate groups and between the first and 2nd phosphate groups. The reason that these bonds are considered "loftier-energy" is because the products of such bail breaking—adenosine diphosphate (ADP) and one inorganic phosphate group (Pi)—accept considerably lower costless energy than the reactants: ATP and a h2o molecule. Considering this reaction takes place with the employ of a water molecule, it is considered a hydrolysis reaction. In other words, ATP is hydrolyzed into ADP in the following reaction:
\[\ce{ATP + H_2O \rightarrow ADP + P_{i} + gratis\: energy} \nonumber\]
Like most chemical reactions, the hydrolysis of ATP to ADP is reversible. The reverse reaction regenerates ATP from ADP + Pi. Indeed, cells rely on the regeneration of ATP just as people rely on the regeneration of spent money through some sort of income. Since ATP hydrolysis releases energy, ATP regeneration must require an input of gratis free energy. The formation of ATP is expressed in this equation:
\[\ce{ADP + P_{i} + costless\: energy \rightarrow ATP + H_2O} \nonumber\]
Two prominent questions remain with regard to the utilise of ATP as an energy source. Exactly how much free free energy is released with the hydrolysis of ATP, and how is that gratuitous free energy used to do cellular work? The calculated ∆K for the hydrolysis of one mole of ATP into ADP and Pi is −7.iii kcal/mole (−30.5 kJ/mol). Since this calculation is true under standard weather condition, it would be expected that a different value exists under cellular weather. In fact, the ∆G for the hydrolysis of one mole of ATP in a living jail cell is almost double the value at standard conditions: xiv kcal/mol (−57 kJ/mol).
ATP is a highly unstable molecule. Unless quickly used to perform piece of work, ATP spontaneously dissociates into ADP + Pi, and the gratuitous energy released during this process is lost as estrus. The second question posed above, that is, how the energy released past ATP hydrolysis is used to perform work inside the cell, depends on a strategy called energy coupling. Cells couple the exergonic reaction of ATP hydrolysis with endergonic reactions, allowing them to proceed. One example of energy coupling using ATP involves a transmembrane ion pump that is extremely important for cellular function. This sodium-potassium pump (Na+/Chiliad+ pump) drives sodium out of the cell and potassium into the cell (Figure \(\PageIndex{2}\)). A large percentage of a cell's ATP is spent powering this pump, because cellular processes bring a neat bargain of sodium into the cell and potassium out of the cell. The pump works constantly to stabilize cellular concentrations of sodium and potassium. In social club for the pump to plow one cycle (exporting three Na+ ions and importing ii Thousand+ ions), one molecule of ATP must be hydrolyzed. When ATP is hydrolyzed, its gamma phosphate doesn't simply bladder abroad, merely is really transferred onto the pump protein. This process of a phosphate group binding to a molecule is called phosphorylation. As with about cases of ATP hydrolysis, a phosphate from ATP is transferred onto another molecule. In a phosphorylated state, the Na+/K+ pump has more free energy and is triggered to undergo a conformational change. This change allows information technology to release Na+ to the outside of the prison cell. It then binds extracellular One thousand+, which, through another conformational change, causes the phosphate to detach from the pump. This release of phosphate triggers the K+ to be released to the inside of the jail cell. Substantially, the energy released from the hydrolysis of ATP is coupled with the free energy required to power the pump and send Na+ and Thousand+ ions. ATP performs cellular piece of work using this basic form of free energy coupling through phosphorylation.
Art Connexion
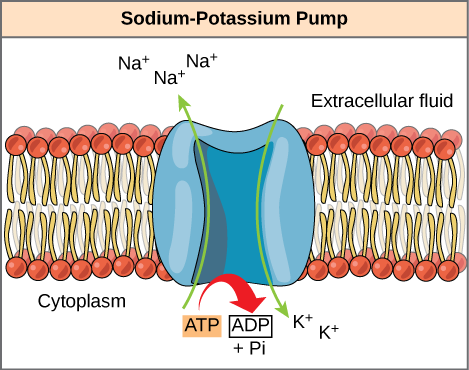
The hydrolysis of one ATP molecule releases seven.iii kcal/mol of energy (∆G = −seven.3 kcal/mol of energy). If it takes two.1 kcal/mol of energy to movement ane Na+ across the membrane (∆G = +2.1 kcal/mol of energy), how many sodium ions could be moved by the hydrolysis of one ATP molecule?
Often during cellular metabolic reactions, such as the synthesis and breakup of nutrients, certain molecules must exist altered slightly in their conformation to go substrates for the next step in the reaction series. One case is during the very first steps of cellular respiration, when a molecule of the sugar glucose is broken downward in the process of glycolysis. In the first step of this process, ATP is required for the phosphorylation of glucose, creating a loftier-energy but unstable intermediate. This phosphorylation reaction powers a conformational change that allows the phosphorylated glucose molecule to exist converted to the phosphorylated sugar fructose. Fructose is a necessary intermediate for glycolysis to move forward. Here, the exergonic reaction of ATP hydrolysis is coupled with the endergonic reaction of converting glucose into a phosphorylated intermediate in the pathway. Once over again, the free energy released by breaking a phosphate bond within ATP was used for the phosphorylation of some other molecule, creating an unstable intermediate and powering an important conformational change.
Link to Learning

See an interactive animation of the ATP-producing glycolysis procedure at this site.
Summary
ATP is the master energy-supplying molecule for living cells. ATP is made upwards of a nucleotide, a v-carbon sugar, and three phosphate groups. The bonds that connect the phosphates (phosphoanhydride bonds) have loftier-energy content. The energy released from the hydrolysis of ATP into ADP + Pi is used to perform cellular work. Cells apply ATP to perform work by coupling the exergonic reaction of ATP hydrolysis with endergonic reactions. ATP donates its phosphate group to some other molecule via a process known every bit phosphorylation. The phosphorylated molecule is at a college-free energy state and is less stable than its unphosphorylated form, and this added energy from the add-on of the phosphate allows the molecule to undergo its endergonic reaction.
Fine art Connections
Figure \(\PageIndex{2}\): The hydrolysis of one ATP molecule releases seven.3 kcal/mol of free energy (∆G = −7.3 kcal/mol of energy). If it takes 2.1 kcal/mol of energy to motion ane Na+ across the membrane (∆Yard = +2.1 kcal/mol of energy), how many sodium ions could exist moved past the hydrolysis of one ATP molecule?
- Answer
-
Three sodium ions could exist moved past the hydrolysis of one ATP molecule. The ∆Thousand of the coupled reaction must be negative. Movement of three sodium ions beyond the membrane will take 6.3 kcal of energy (two.one kcal × 3 Na+ ions = 6.3 kcal). Hydrolysis of ATP provides seven.3 kcal of energy, more than enough to power this reaction. Motility of 4 sodium ions beyond the membrane, all the same, would require 8.4 kcal of free energy, more than than 1 ATP molecule can provide.
Glossary
- ATP
- adenosine triphosphate, the jail cell's energy currency
- phosphoanhydride bond
- bond that connects phosphates in an ATP molecule
Contributors and Attributions
-
Connie Rye (East Mississippi Community Higher), Robert Wise (University of Wisconsin, Oshkosh), Vladimir Jurukovski (Suffolk County Customs College), Jean DeSaix (University of North Carolina at Chapel Hill), Jung Choi (Georgia Institute of Technology), Yael Avissar (Rhode Isle College) amongst other contributing authors. Original content by OpenStax (CC BY 4.0; Download for costless at http://cnx.org/contents/185cbf87-c72...f21b5eabd@9.87).
Source: https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(OpenStax)/2%3A_The_Cell/06%3A_Metabolism/6.4%3A_ATP%3A_Adenosine_Triphosphate
0 Response to "when a high-energy bond of atp is broken, primarily what happens to the released energy?"
Post a Comment